The new ELIGARD® pre-connected syringe system is a user-friendly device with an intuitive and simple way of use requiring only 3 stages to prepare the product.1-4
Furthermore, the trusted ATRIGEL delivery system ensures a reliable and sustained leuprorelin acetate release.5-7
References+
- 1. Data on file, Recordati 2022 (Tolmar: ELIGARD® (leuprolide acetate) for injectable suspension, 45 mg prescribing information. Fort Collins, CO: Tolmar; 2022).
- 2. ELIGARD® 7.5 mg Summary of Product Characteristics.
- 3. ELIGARD® 22.5 mg Summary of Product Characteristics.
- 4. ELIGARD® 45 mg Summary of Product Characteristics.
- 5. Data on file, Recordati 2023.
- 6. Sartor O. Eur Urol Suppl 2006; 5: 905-10.
- 7. Merseburger AS, Roesch MC. Expert Rev Anticancer Ther. 2022;22(7):703-715. doi:10.1080/14737140.2022.2082947
ELIGARD® new pre-connected syringe system preparation
Allow the product to come to room temperature by removing from the refrigerator approximately 30 minutes before use. Prepare the patient for injection first, followed by preparation of the product, using the following instructions.1-3
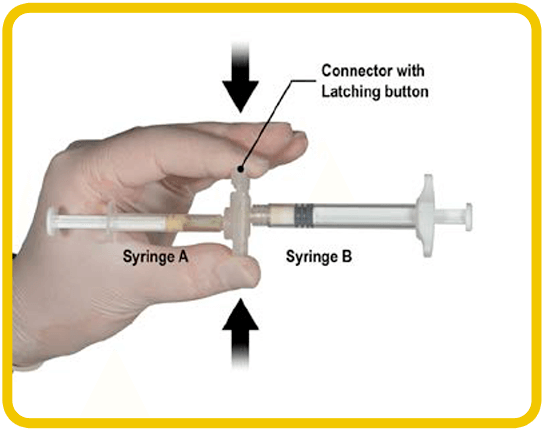
OPEN AND CLICK1-3
1. On a clean field, open the tray by peeling back the foil from the corner. Discard the desiccant pack(s). Remove the ELIGARD® pre-connected syringe system from the tray. Syringe A and Syringe B should not be aligned yet.
2. Hold the latching button on the connector between your finger and thumb and press until you hear a click. The two syringes should now be aligned.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.
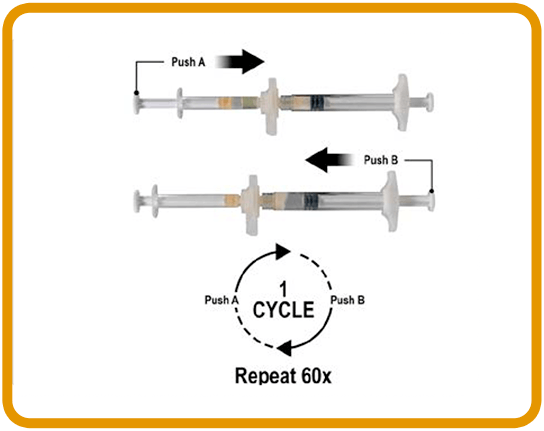
MIX1-3
3. Holding the syringes in a horizontal position, transfer the liquid contents of Syringe A into the leuprorelin acetate powder contained in Syringe B. Thoroughly mix the product for 60 cycles by gently pushing the contents of both syringes back and forth between both syringes (a cycle is one push of the plunger for Syringe A and one push of the plunger for Syringe B) in a horizontal position to obtain a homogenous, viscous solution.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.
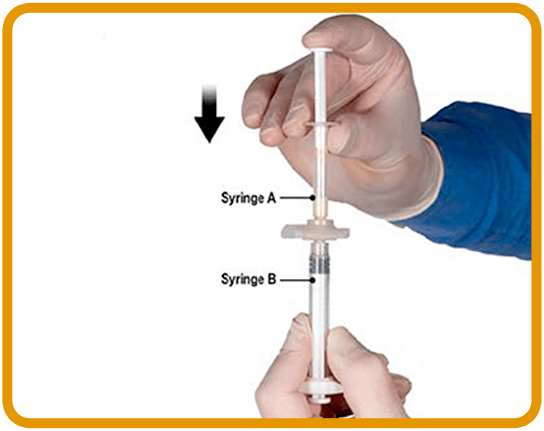
TRANSFER1-3
4. After mixing, hold the syringes vertically with Syringe B on the bottom. The syringes should remain securely coupled. Draw the entire mixed product into Syringe B (short, wide syringe) by pushing down the Syringe A plunger and slightly withdrawing the Syringe B plunger.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.
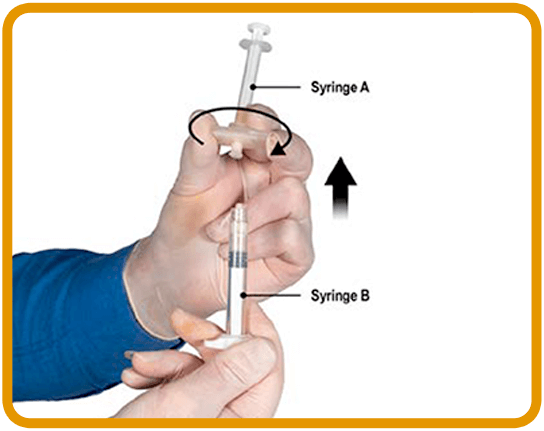
DETACH1-3
5. While ensuring Syringe A plunger is fully pushed down, hold the connector and unscrew it from Syringe B. Syringe A will remain attached to the connector. Ensure that no product leaks out as the needle will not be properly secured when attached.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.
ADMINISTRATION1-3
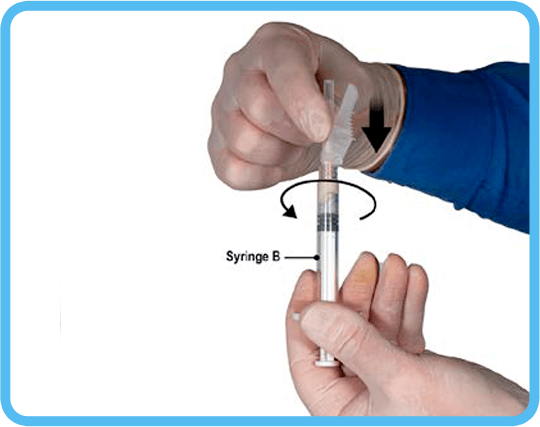
6. Secure the safety needle to Syringe B by holding the syringe and gently turning the needle clockwise with approximately a three-quarter turn. Do not over-tighten as this may crack the needle hub and cause the product to leak out during injection. In the event of damage to the needle hub, a new replacement product should be used.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.
ADMINISTRATION1-3
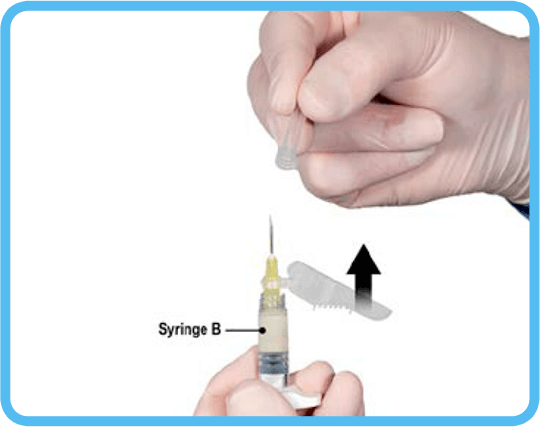
7. Move the safety shield away from the needle and remove the protective needle cover immediately prior to administration. Important: Do not operate the safety needle mechanism before administration. Should the needle hub appear to be damaged, or leak, the product should NOT be used. The damaged needle should NOT be replaced and the product should NOT be injected. In the event of damage to the needle hub, use another ELIGARD® kit.
References+
- 1. Eligard® 7.5 mg Summary of Product Characteristics.
- 2. Eligard® 22.5 mg Summary of Product Characteristics.
- 3. Eligard® 45 mg Summary of Product Characteristics.